The sensitivity of electronic balance is 0.3 mg
Converting 0.3 mg to g:
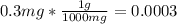 g
g
So, the mass of a substance weighed on the balance can be measured accurately up to four decimal places.
Given the mass of sample is 1.2300 g. The number of significant figures in the measurement will be 5 because the balance can measure the mass of a sample accurately up to four decimal points, all the digits in the measurement will be significant.