Correct answer: d) 17
The chemical formula of pentane is
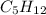
The subscript of each element represents the number of moles of atoms of that element present in one formula unit of the molecule.
Pentane has carbon and hydrogen atoms. The subscript of C is 5 indicating that each mole of pentane has 5 moles of carbon atoms. Similarly the subscript on hydrogen is 12 indicating that each mole of pentane has 12 moles of hydrogen atoms.
Therefore, the total number moles of atoms in one mole pentane = 5 C +12 H = 17