The balanced chemical equation between phosphorus and oxygen can be given as,
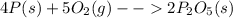
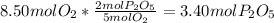
3.80 mol
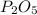 is formed from P (limiting reactant):
is formed from P (limiting reactant):
Moles of P =
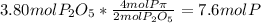
3.40 mol
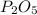 is produced when Oxygen is limiting:
is produced when Oxygen is limiting:
Moles of
 =
=
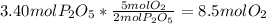
So we have 7.6 mol P and 8.5 mol O2.
Calculating the moles of P2O5 formed from the given moles of P and O2:
Moles of phosphorus pentoxide from P =
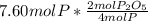
So the moles of
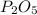 produced from P are
produced from P are
=
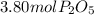
Moles of phosphorus pentoxide formed from O2 =
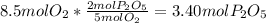
O2 produced the least amount of the product so oxygen would be the limiting reactant.
Moles of
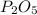 produced will be 3.40 mol
produced will be 3.40 mol