The balanced chemical equation representing the neutralization reaction of hydrochloric acid (HCl) and sodium hydroxide (NaOH),
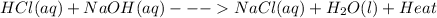
This reaction is exothermic that is it proceeds by the release of heat energy. This heat energy is lost to the surroundings. This is the reason for the warming up of the beaker where the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) takes place. The enthalpy of such reaction will be negative as the reaction is exothermic.