partial pressure in a mixture of two or more gases will be given by formula
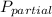 = mole fraction of gas * total pressure
= mole fraction of gas * total pressure
now here mole fraction is same as percentage of gas in the mixture
Now mole fraction of oxygen is 0.20095 (20.095%)
now here pressure of oxygen in the mixture is given as
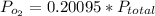
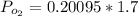
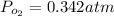
so pressure due to oxygen in the mixture will be 0.342 atm