Given equation :
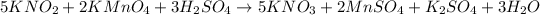
Given information = 2.47 grams KNO2 and excess KMnO4 and we need to find grams of water (H2O).
Since KMnO4 is in excess, so grams of water(H2O) can be calculated using grams of KNO2 with the help of stoichiometry.
To find grams of water(H2O) from grams of KNO2 , we need to follow three steps.
Step 1. Convert 2.47 grams of KNO2 to moles of KNO2.
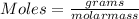
Molar mass of KNO2 = 85.10 g/mol
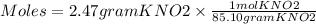
Moles = 0.0290 mol KNO2
Step 2. Convert moles of KNO2 to moles of H2O using mole ratio.
Mole ratio are the coefficient present in front of the compound in the balanced equation.
Mole ratio of KNO2 : H2O is 5 : 3 (5 coefficient of KNO2 and 3 coefficient of H2O)
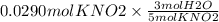
Mole = 0.0174 mol H2O
Step 3. Convert mole of H2O to grams of H2O
Grams = Moles X molar mass
Molar mass of H2O = 18.00 g/mol
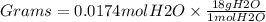
Grams of water = 0.313 grams H2O
Summary : The above three steps can also be done in a singe setup as shown below.
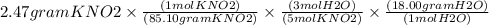
In the above setup similar units get cancelled out and we will get grams of H2O as 0.313 grams water (H2O)