So to find the molar mass of NaCl, we need to add both the molar weight for sodium (Na) and chlorine (Cl) from the periodic table:
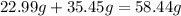
This is the molar weight of table salt, NaCl.
So we are told we have 7.2 moles of this substance. Then we can set up an equation, cancel units, and solve for the amount of grams that this is:
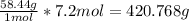
So now we know that 7.2 mol of NaCl is equal to 420.77g.