Answer: -
0.2 moles of NaCl are contained in 100.0 mL of a 2.0 M solution
Explanation: -
Strength of NaCl = 2.0 M
Volume of NaCl solution = 100.0 mL
=
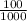
= 0.1 L
Number of moles of NaCl = volume of NaCl x Strength of NaCl
= 0.1 L x 2.0 M
= 0.2 moles
0.2 moles of NaCl are contained in 100.0 mL of a 2.0 M solution