Answer: -
If a tank of gasoline contains 80 liters and that its density is 0.77 kg/liter, 0.26 kg of CO₂ are produced for each tank of gasoline burned.
Explanation: -
Density of the gasoline = 0.77 kg / liter
Volume of the tank containing the gasoline = 80 liter.
Mass of gasoline produced from each tank
= Volume of the tank containing the gasoline x Density of the gasoline
=
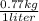 x 80 liter
x 80 liter
= 61.6 kg
Chemical formula of gasoline = C₈H₁₈
Molar mass of gasoline C₈H₁₈ = 12 x 8 + 1 x 18 = 114 g/ mol
Number of moles of C₈H₁₈ =
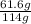 x 1 mol
x 1 mol
= 0.54 mol of C₈H₁₈
The chemical equation for the burning of gasoline is
2 C₈H₁₈ + 25 O₂ → 16 CO₂ + 18 H₂O
From the balanced equation we see
2 mol of C₈H₁₈ gives 16 mol of CO₂
0.54 mol of C₈H₁₈ gives
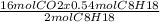 mol of CO₂
mol of CO₂
= 4.32 mol of CO₂
Molar mass of CO₂ = 12 x 1 + 16 x 3 =60 g / mol
Mass of CO₂ = Molar mass of CO₂ x Number of moles of CO₂
=
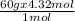
= 259.2 g
=
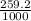
= 0.259 Kg
= 0.26 kg rounded off to 2 significant figures.
Thus if a tank of gasoline contains 80 liters and that its density is 0.77 kg/liter, 0.26 kg of CO₂ are produced for each tank of gasoline burned.