Given the half life of the first order decomposition reaction is 355 s
Rate constant of the first order reaction is related to the half life by the equation,
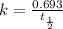
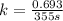
k = 0.00195
The concentration of the substance is decreased to 1/4 th.
If we start with 1 M solution, after time t the concentration becomes 1/4th = 0.25 M
First order rate law:
![[A] = [A]_(0) e^(-kt)](https://img.qammunity.org/2019/formulas/chemistry/college/2z5oibr6vmkdrx8ys70glubly7b8wyeokc.png)
![[A] = 0.25 M](https://img.qammunity.org/2019/formulas/chemistry/college/bsbsai05n201o729nl7ct3ots8pkwmrn7h.png)
![[A]_(0) = 1 M](https://img.qammunity.org/2019/formulas/chemistry/college/10rl7uuy69k0fpadg1ic65xbywp1hzs961.png)
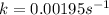
Plugging in the values to solve for t,
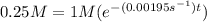
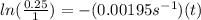
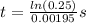
t = 710 s