Step-by-step explanation:
According to rate law, rate of a reaction depends upon the concentration of the species participating in the reaction.
For example,
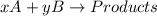
![Rate \propto k[A]^(x)[B]^(y)](https://img.qammunity.org/2019/formulas/chemistry/high-school/41wena40cirqrxbewueddbq5ztef51mq0f.png)
Rate =
![k[A]^(x)[B]^(y)](https://img.qammunity.org/2019/formulas/chemistry/high-school/k46y3wx2zft358jro79w6er36roen2w738.png)
Therefore, when there is increase in concentration of reactants then there will be more number of collisions. Due to this there will rapid formation of products. Hence, rate of reaction will increase.
Whereas decrease in concentration of reactants will decrease the rate of a reaction.