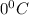When water at 50 C is added to ice at -12 C, heat is transferred from hot water to ice.
- Heat given out by water = Heat absorbed by ice
Calculating the heat released by hot water:
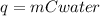 ΔT
ΔT
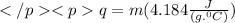

Calculating heat absorbed by 16 g of ice: Ice at
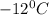 is converted to ice at
is converted to ice at
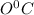 and then ice at
and then ice at
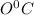 to water at
to water at
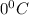
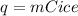 ΔT +
ΔT +
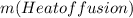
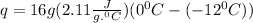 +
+
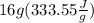
q = 405.12 J +5336.8 J =5741.92 J
- Heat given out by water = Heat absorbed by ice
-(
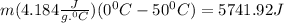
m = 27.4 g
Therefore, 27.4 g water at
 must be added to 16 g of ice at
must be added to 16 g of ice at
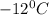 to convert to liquid water at
to convert to liquid water at