Answer: The volume of water vapor produced will be 3 L.
Step-by-step explanation:
At STP:
1 mole of a gas occupies 22.4 liters of volume.
For the reaction of combustion of methane, the equation follows:
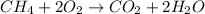
By stoichiometry of the reaction:
If
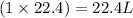 of methane gas produces
of methane gas produces
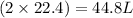 of water vapor.
of water vapor.
Then, 1.5 L of methane gas will produce =
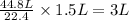 of water vapor.
of water vapor.
Hence, the volume of water vapor produced will be 3 L.