Mass of Copper metal dissolved = 0.1090 g
Moles of Copper dissolved =
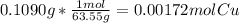
Total volume of the solution = 203.1 mL
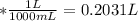
Calculating the molarity of copper ions in the solution:
Molarity of a solution =
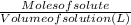
=
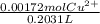
=0.00845 mol/L
Therefore the molarity of copper ions is 0.00845 mol/L