Given information : Free-base form of cocaine solubility 1.00 g in 6.70 mL ethanol.
We have to find molarity.
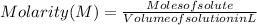
To find molarity we need to first find moles of solute and volume of solution.
In our question solute is Cocaine(C17H21NO4) and ethanol (CH3CH2OH) is solvent.
Volume of solution = Volume of solute + Volume of solvent.
In our question , density of cocaine is not given that means amount of cocaine is very less as compared to that of solvent , so to find the volume of solution we can only consider volume of solvent that means we can take volume of solution as 6.70 mL.
Moles of solute cocaine (C17H21NO4) =
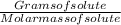
Molar mass of coacine (C17H21NO4) = 303.358 g/mol
Gram of solute given = 1 gram
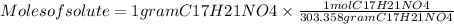
Moles of solute C17H21NO4 = 0.00330 mol C17H21NO4
Volume of solution should be in L , so we will convert 6.70 mL to L
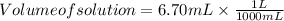
Volume of solution = 0.0067 L
Now we can find molarity by plugging the moles of solute and volume of solution value in the formula.
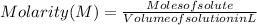
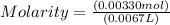
Molarity = 0.49 mol/L or 0.49 M