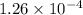Answer: The concentration of hydronium ions in a solution is
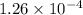
Step-by-step explanation:
pH is defined negative logarithm of hydrogen or hydronium ion concentration present in a solution. It is basically defined as the power of hydrogen ions in a solution.
Mathematically,
![pH=-\log[H^+]](https://img.qammunity.org/2019/formulas/chemistry/high-school/vwilut25e4cux34589pwoorivy6w6y51xe.png)
We are given:
pH of the solution = 3.9
Putting values in above equation, we get:
![3.9=-\log[H^+]](https://img.qammunity.org/2019/formulas/chemistry/high-school/j9nco89cfxwrbgf7nlegvw479h0z8xvp3c.png)
![[H^+]=antilog(-3.9)](https://img.qammunity.org/2019/formulas/chemistry/high-school/qdhrvdxc876yxpez511y516mfa0bygp1i0.png)
![[H^+]=1.26* 10^(-4)](https://img.qammunity.org/2019/formulas/chemistry/high-school/tv5o5fokanwkswfpkjt06zr0ytcqrit7pm.png)
Hence, the concentration of hydronium ions in a solution is