The balanced chemical equation for the reaction given is:
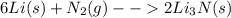
Moles of
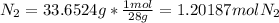
Moles of Li =
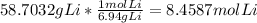
Determining the limiting reagent: The reactant that gives least amount of the product will be the limiting reactant.
Mass of
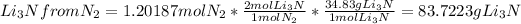
Mass of
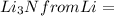
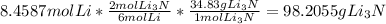
 will be the limiting reactant and the mass of lithium nitride produced will be dependent on the mass of limiting reactant.
will be the limiting reactant and the mass of lithium nitride produced will be dependent on the mass of limiting reactant.
Therefore, mass of lithium nitride produced = 83.7223 g