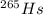Here in all nuclear reactions we can say that mass conservation and charge conservation is always true
Here iron nuclei smashed into lead nuclei and then a new nuclei will form which will released along with a neutron
Now in this reaction mass and charge will remain conserved
mass number of iron + mass number of lead = mass number of new nuclei + mass number of neutron
58 + 208 = x + 1
x = 265
so the new nuclei formed will have mass number A = 265
now we will use charge conservation
Number of protons in iron + number of protons in lead = number of protons in new nuclei + number of protons in neutron
26 + 82 = z + 0
z = 108
so the new nuclei will form with atomic number z = 108 and mass number A = 265
If we refer periodic table to find such atom we will see that this is
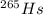
so the new nuclei formed is