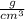Density of a substance can be defined as the mass of that substance per unit volume.
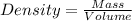
Given the mass of object = 9.0 g
Volume of the object will be the volume of water displaced by the object
So, volume of the object =
Calculating the density from mass and volume:
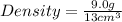
= 0.69
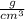
Therefore, the density of given object is 0.69