Answer: -
13.545
Explanation: -
Let the volume of the solution be 1000 mL
Density = 1.04 g/ mL
Mass = density x vol
= 1.04 g/ mL x 1000 mL
= 1040 g
Amount of NaOH =
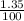 x 1040 g
x 1040 g
= 14.04 g
Molar mass of NaOH = 23 x 1 + 16 x 1 + 1 x 1
= 40 g / mol
Number of moles = Amount of NaOH / Molar mass
= 14.04 /40
= 0.351 mol
So 1000 ml of the solution has 0.351 mol
Molarity of NaOH = 0.351 M
Using the relation
pOH = - log [OH-]
= - log 0.351
= 0.455
Using the relation pH + pOH = 14
pH = 14 - pOH
= 14 - 0.455
= 13.545