Answer: -
16.39 mL is the minimum volume of boiling water needed to dissolve 0.200 g of phenacetin
Explanation: -
Mass of phenacetin = 0.200 g
The solubility in boiling water of phenacetin is 1.22 grams of solute per 100 milliliters of water.
Thus, minimum volume of boiling water required to dissolve 1.22 g is 100 mL
Minimum volume of boiling water required to dissolve 0.200 g is
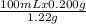
= 16.39 mL