A liquid takes 10.14 x 10^6 J of energy to boil 28.47 kg at 298 K. Using the Latent heats of vaporization of some of the substances:
Acetone: 538,900
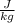
Ammonia: 1,371,000
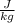
Propane: 356,000
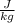
Methane: 480,600
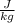
Ethanol: 841,000
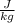
Calculating the latent heat of vaporization of the given substance:
Heat required =

Mass of the substance boiled = 28.47 kg
Latent heat of vaporization =
So the substance boiled is propane.