Answer:- 117 mL of HCl are used.
Solution:- The balanced equation for the reaction of HCl with barium hydroxide is written as:
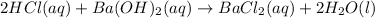
From above equation, HCl and
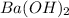 react in 2:1 mol ratio.
react in 2:1 mol ratio.
We will calculate the moles of barium hydroxide on dividing its grams by its molar mass.
Molar mass of Barium hydroxide is given as 171.3 g per mol.
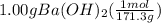
= 0.00584 mol
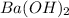
Using mol ratio we calculate the moles of HCl as:
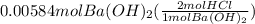
= 0.01168 mol HCl
We know that molarity is moles of solute per liter of solution. We have 0.01168 moles of HCl and its molarity is 0.100 M. So, we can calculate the liters of HCl solution used on dividing the moles by molarity as and on multiplying by 1000 the liters are converted to mL since, 1 L = 1000 mL.
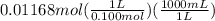
= 116.8 mL
It could be round to 117 mL.
So, 117 mL of HCl are required.