Answer:84.6 kJ of energy released when 2.50 moles of nitrogen dioxide is decomposed.
Step-by-step explanation:
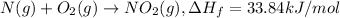
Energy released when 1 mole of
 is formed = 33.84 kJ
is formed = 33.84 kJ
And When 1 mole of
 is decomposed it gives out 33.84 kJ of energy.
is decomposed it gives out 33.84 kJ of energy.
Energy released when 2.50 moles of nitrogen dioxide is decomposed:
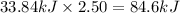
84.6 kJ of energy released when 2.50 moles of nitrogen dioxide is decomposed.