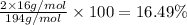Step-by-step explanation:
Molecular mass of carbon caffeine(
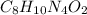 ), = 194 g/mol
), = 194 g/mol
Atomic mass of carbon = 12 g/mol
Atomic mass of hydrogen atom = 1 g/mol
Atomic mass of nitrogen atom = 1 g/mol
Atomic mass of oxygen atom = 1 g/mol
Percentage of an element in a compound:
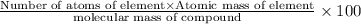
Percentage of carbon:
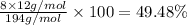
Percentage of hydrogen:
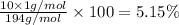
Percentage of nitrogen:
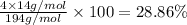
Percentage of oxygen :