Answer:
The pH of the nitric acid solution is 3.70.
Step-by-step explanation:
The pH is negative logarithm of hydrogen ion concentration.
![pH=-\log[H^+]](https://img.qammunity.org/2019/formulas/chemistry/high-school/vwilut25e4cux34589pwoorivy6w6y51xe.png)
Concentration of nitric acid =
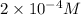
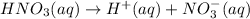
1 mole of nitric acid gives 1 mole of hydrogen ions.
Then
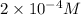 of nitric acid will give .
of nitric acid will give .
![[H^+]=1* 2* 10^(-4) M=2* 10^(-4) M](https://img.qammunity.org/2019/formulas/chemistry/middle-school/dj01y1x8qwtjeocejm0mx1mld3lgsbww1h.png)
The pH of the solution :
![pH=-\log[2* 10^(-4) M]=3.70](https://img.qammunity.org/2019/formulas/chemistry/middle-school/ghtdq6m5r7xom72p3ry6qkevk3ge333x9a.png)