Answer:
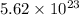 formula units of sodium hydroxide constitutes 0.934 moles.
formula units of sodium hydroxide constitutes 0.934 moles.
Step-by-step explanation:
Number of molecules of sodium hydroxide =
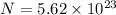 molecules
molecules
Moles of sodium hydroxide = n
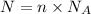
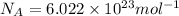
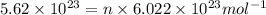
n = 0.934 moles
So,
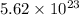 formula units of sodium hydroxide constitutes 0.934 moles.
formula units of sodium hydroxide constitutes 0.934 moles.