So we know that chromium has a 3+ charge on it (
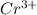 ) and oxygen has a 2- charge (
) and oxygen has a 2- charge (
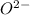 .
.
In order to create a neutral compound, the charges on these atoms must cancel each other out. The best way to do this quickly is to take the charge of the other atom and make it the subscript for the atom of interest.
For example, lets look at chromium. The oxygen has a charge of 2-, so we're going to make the amount of chromium in the new compound with a subscript two: (
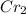 )
)
Now look at oxygen. The chromium has a charge of 3+, so we're going to make the amount of oxygen in the new compound with a subscript three: (
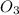 )
)
Then we put them together to make the new neutral compound:
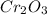
So when we look at this ratio, we see that Cr:O must be 2:3 in order to remain a neutral compound.