Answer: -
9270 grams
Explanation: -
Number of molecules of C₆H₁₂O₆ = 3.25 x 10 ²⁶
According to Avogadro's law,
6.02 x 10 ²³ molecules of C₆H₁₂O₆ are present in 1 mole of C₆H₁₂O₆
3.25 x 10 ²⁶ molecules of C₆H₁₂O₆ are present in
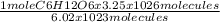 of C₆H₁₂O₆
of C₆H₁₂O₆
= 54 mol of C₆H₁₂O₆
Molar mass of C₆H₁₂O₆ = 12 x 6 + 1 x 12 + 16 x 6 = 180g /mol
Mass of C₆H₁₂O₆ = 54 mol x 180g /mol
= 9270 g