Answer:
The option B is a correct answer.
Step-by-step explanation:
Mass of aluminum ethoxide = 350 g
Moles of aluminum ethoxide =
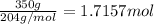
In 1 mole of aluminum ethoxide there are 6 moles of oxygen atom.
Then 1.7157 moles of aluminum ethoxide will have :
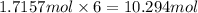
Mass of 10.291 mole of oxygen atom = 10.294 mol 16 g/mol = 164.70 g[/tex]
The closest option to our answer is option B.
Hence, option B is correct answer.