q = mcΔT
where, q = heat required in Joules
m = mass in grams
c = specific heat in joules/gram °C
ΔT = change in temperature = Final temperature(T₂)-Initial Temperature (T₁)
Given, q=267.3 kJ=267300 Joules
m=18kg=18000 gram
ΔT=318-285= 33K = 33°C
Rearranging formula, c=
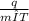
c=
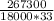
c=0.45 joules/gram °C