Answer: -
1) Concentration of NaOH = 2.0 x 10⁻² M
Since NaOH is a monoacidic base,
The Hydroxide OH⁻ concentration will be the same as concentration of NaOH.
Concentration of hydroxide = [OH⁻] = 2.0 x 10⁻² M
We know the ionic product of water = 1.0 x 10⁻¹⁴
[H₃O⁺][OH⁻] = 1.0 x 10⁻¹⁴
Thus hydronium ion concentration [H₃O⁺] =
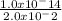
= 5.0 x 10⁻¹³ M
2) Concentration of HNO₃ = 5.0 x 10⁻⁴ M
Since HNO₃ is a monobasic acid,
[H₃O⁺] = 5.0 x 10⁻⁴ M
pH = - log [H₃O⁺] = - log [5.0 x 10⁻⁴]
= 3.3
3) Concentration of Sr(OH)₂ = 3.45 x 10⁻² M
Since Sr(OH)₂ is a diacidic base,
Concentration of OH⁻ = [OH⁻] = 2 x 3.45 x 10⁻² M
We know the ionic product of water = 1.00 x 10⁻¹⁴
[H₃O⁺][OH⁻] = 1.0 x 10⁻¹⁴
Thus hydronium ion concentration [H₃O⁺] =
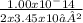
= 1.45 x 10 ⁻¹³ M
pH = - log [H₃O⁺] = - log [1.45 x 10 ⁻¹³]
= 12.8
4) pH = 7.0
We know that [H₃O⁺] =
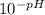
= 1.0 x 10⁻⁷ M
5) pH = 5.00
We know that [H₃O⁺] =
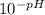
= 1.00 x 10⁻⁵ M
We know the ionic product of water = 1.0 x 10⁻¹⁴
[H₃O⁺][OH⁻] = 1.00 x 10⁻¹⁴
[OH⁻] =
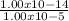
= 1.00 x 10⁻⁹ M