Drysol is a mixture of aluminum chloride hexahydrate and an alcohol base.
Mass of aluminum chloride hexahydrate = 15 g
Assuming the density of alcohol base to be 1 g/mL
Mass of alcohol base =
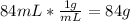
Total mass of the mixture = 15 g + 84 g = 99 g
Mass % of the mixture =
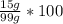
= 15.2 %