Answer: -
60 mL of potassium chloride 20% should be diluted to prepare 480 ml of potassium chloride 2.5%
Explanation: -
Volume of potassium chloride solution = 480 mL
Percentage of potassium chloride = 2.5%
Amount of Potassium chloride =
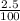 x 480 mL
x 480 mL
= 12 mL
Let the required amount be R.
20 % of R = 12 mL.
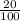 x R = 12mL
x R = 12mL
R = 60 mL.
Thus, 60 mL of potassium chloride 20% should be diluted to prepare 480 ml of potassium chloride 2.5%