Answers: -
8.75 L of O₂
Explanation: -
Molar mass of KClO₃ = 39 x 1 + 35.5 x 1 + 16 x 3 = 122.5 g
The balanced chemical equation for this process is
2KClO₃(s) --> 2KCl (s) + 3O₂(g)
From the balanced chemical equation we see
2 mol of KClO₃ gives 3 mol of O₂
At STP,
2 x 122.5 g of KClO₃ gives 3 x 22.4 L of O₂
31.9 g of KClO₃ gives 3 x 22.4 L of O₂ x
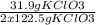
= 8.75 L of O₂