Answer: -
81.03 g magnesium oxide is formed when 48.62g of magnesium is reacted with excess oxygen
Explanation: -
Atomic mass of Mg = 24 g
Molar mass of MgO = 24 x 1 + 16 x 1
= 40 g
The balanced chemical equation for this reaction is
2 Mg + O₂ → 2MgO
From the balanced chemical equation we see
2 mol of Mg gives 2 mol of MgO.
2 x 24 g of Mg gives 2 x 40g of MgO.
48.62 g of Mg gives
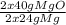 x Mg
x Mg
= 81.03g of MgO