Answer:- The amount of barium phosphate that can be produced is 2.87 g.
Solution:- It's a stoichiometry problem. So, we need to write the balanced equation and do the calculations with the help of that.
the reaction is double replacement and takes place as:
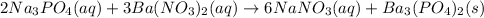
Given, mass of sodium phosphate = 4.50 g
mass of barium nitrate = 3.75 g
Let's calculate the moles for both of these.
moles of sodium phosphate =
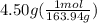
moles of sodium phosphate = 0.0274
moles of barium nitrate =
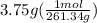
moles of barium nitrate = 0.0143
Now we will calculate the grams of barium phosphate using the calculated moles of each reactant and see which one gives limited amount of it.
Calculations for grams of barium phosphate from moles of sodium phosphate:
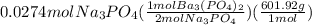
= 8.25 g
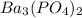
Calculations for grams of barium phosphate from moles of barium nitrate:
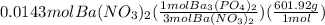
= 2.87 g
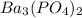
Barium nitrate gives the limited amount of barium phosphate. So, the amount of barium phosphate that can be produced is 2.87 g.