The question is incomplete.
The complete question probably is
The pH scale for acidity is defined by pH = − log₁₀[H⁺] where [H⁺] is the concentration of hydrogen ions measured in moles per liter (M). A solution has a pH of 2.55. Find the hydrogen ion concentration.
Answer: -
0.003 M
Explanation: -
pH = − log₁₀[H⁺]
Thus the hydrogen ion concentration [H⁺] =
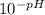
=
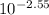
= 0.003 M