Answer: -
5.376 x 10²³ atoms of nickel are in 52.4g of nickel.
Explanation: -
Mass of nickel = 52.4 g
Atomic mass of nickel = 58.69 g / mol
Number of moles of nickel = Mass of nickel / atomic mass
=
![\frac{52.4 g{58.69 g / mol}]()
= 0.893 mol of Nickel.
According to Avogadro's Law,
1 mol of Nickel has 6.02 x 10²³ atoms of nickel.
0.893 mol of nickel has
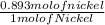 x 6.02 x 10²³ atoms of Nickel
x 6.02 x 10²³ atoms of Nickel
= 5.376 x 10²³ atoms of nickel.
5.376 x 10²³ atoms of nickel are in 52.4g of nickel.