The given dehydration equation is,
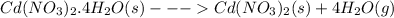
Cadmiumnitrate tetrahydrate when heated dehydrates releasing the combined water as water vapor. The reaction produces 4 moles of gaseous product water vapor. So, the degree of disorder or randomness increases. Hence, the sign of change in entropy is positive.
This reaction is spontaneous at room temperature even if it is endothermic as the sign of change in entropy is positive.