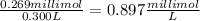Amount of silver nitrate taken = 269.μmol
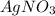
Volume of the solution = 300. mL
Concentration of a solution is generally expressed in terms of molarity. Molarity is defined as the moles of a substance present per liter of the solution.
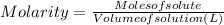
We want the concentration in millimoles/L.
Converting μmol to millimol solute:
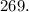 μ
μ
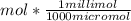 = 0.269 millimol
= 0.269 millimol
Volume from mL to L:
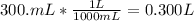
Therefore concentration of the chemist's solution =