Answer: -
24.24 mL
Explanation: -
Mass of CaCO₃ = 1.212 g
Molar mass of CaCO₃ = 40 x 1 +12 x 1 + 16 x 3 = 100 g / mol
Number of moles of CaCO₃ =
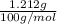
= 0.01212 mol
The balanced chemical equation for the reaction that occurs when CaCO₃ is added to HCl is
CaCO₃ + 2HCl --> CaCl₂ + H₂O + CO₂
From the equation we see
1 mol of CaCO₃ reacts with 2 mol of HCl
0.01212 mol of CaCO₃ react with
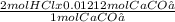
= 0.02424 mol of HCl
Strength of HCl = 1 M
Volume of HCl required = Number of moles of HCl / strength of HCl
= 0.02424 mol / 1 M
= 0.02424 L
= 0.02424 x 1000 mL
= 24.24 mL