The enthalpy change of the reaction when sodium hydroxide and sulfuric acid react can be calculated using the mass of solution, temperature change, and specific heat of water.
The balanced chemical equation for the reaction can be represented as,

Given volume of the solution = 101.2 mL + 50.6 mL = 151.8 mL
Heat of the reaction, q =
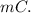 Δ
Δ
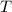
m is mass of the solution = 151.8 mL *
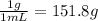
C is the specific heat of solution = 4.18
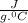
ΔT is the temperature change =
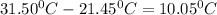
q =
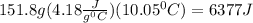
Moles of NaOH =
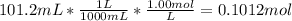 NaOH
NaOH
Moles of
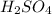 =
=
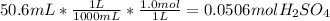
Enthalpy of the reaction =