According to the Avogadro's law, equal volumes of all gases contain equal number of moles under the same conditions of temperature and pressure.
Here the initial volume,
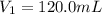
Initial moles of the gas,
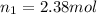
Final volume of the gas
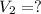
Final moles of the gas
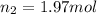
Plugging in the Avogadro's equation,
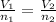
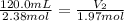
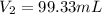
Therefore the 1.97 mol of the gas occupies 99.33 mL at constant temperature and pressure.