Answer: -
1.34 L
Explanation: -
Initial Pressure P 1 = 39.1 bar
Initial Temperature T 1 = 643 K
Let the initial volume be V 1.
Final pressure P 2 = 87.0 bar
Final temperature T 2 = 525 K.
Final volume V 2 = 0.492 L
Using the equation
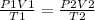
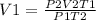
Plugging in the values
We have
V 1 = 87 bar x 0.492 L x 643 K / (39.1 bar x 525 K)
= 1.34 L
Thus, a gas is contained in a thick-walled balloon. When the pressure changes from 39.1 bar to 87.0 bar the volume changes from 1.34 L to 0.492L and the temperature changes from 643K to 525K