Answer: -
Thus 7.2 moles of CO₂ are produced when 3.6 moles of C₂H₂ reacts.
Explanation: -
C₂H₂ reacts with O₂ to produce CO₂ and H₂O
The balanced chemical equation for the reaction is
2 C₂H₂ + 5 O₂→ 4 CO₂ + 2 H₂O
From the balanced equation we can see that
2 moles of C₂H₂ gives 4 moles of CO₂
3.6 moles of C₂H₂ gives
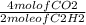 x 3.6 moles of C₂H₂
x 3.6 moles of C₂H₂
= 7.2 moles of CO₂
Thus 7.2 moles of CO₂ are produced when 3.6 moles of C₂H₂ reacts.