Answer : The correct option is, (C) 3,084,840.0 J
Explanation :
Formula used :
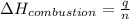
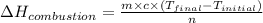
where,
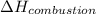 = enthalpy of combustion = ?
= enthalpy of combustion = ?
m = mass of water = 328 g
 = specific heat of water=
= specific heat of water=
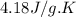
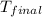 = final temperature = 343 K
= final temperature = 343 K
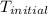 = initial temperature = 298 K
= initial temperature = 298 K
n = moles of butane = 0.02 mole
Now put all the given values in the above formula, we get enthalpy of combustion.
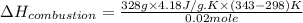
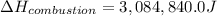
Therefore, the enthalpy of combustion is, 3,084,840.0 J