The new pressure would be = 4.46 atm
Further explanation
Given
V₁=6.7 L(at STP, 1 atm 273 K)
V₂=1.5 L
Required
The new pressure
Solution
Boyle's Law
At a constant temperature, the gas volume is inversely proportional to the pressure applied
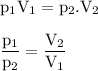
P₂ = (P₁V₁)/V₂
P₂ = (1 atm x 6.7 L)/1.5 L
P₂ = 4.46 atm