Given information : Volume of HCl = 32.40 mL
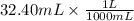
Volume of HCl = 0.0324 L
Concentration of HCl = 0.185 M or 0.185 mol/L (M = mol/L)
Volume of Ca(OH)2 = 27.0 mL
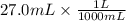
Volume of Ca(OH)2 = 0.027 L
We need to find the concentration of Ca(OH)2.
To find the concentration of Ca(OH)2 we need moles and volume of Ca(OH)2.
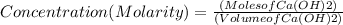
Moles of Ca(OH)2 can be calculated using stoichiometry and volume of Ca(OH)2 is already given to us.
Step 1 : Find the moles of HCl using its given volume and concentration.
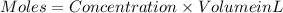
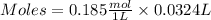
Moles of HCl = 0.005994 mol HCl
Step 2 : We need to find moles of Ca(OH)2 using mol of HCl with the help of mole ratio.
Mole ratio are the coefficient present in front of the compound in a balanced equation.
Mole ratio of Ca(OH)2 : HCl = 1:2 ( 1 coefficient of Ca(OH)2 and 2 coefficient of HCl)
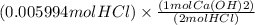
Moles of Ca(OH)2 = 0.002997 mol Ca(OH)2
Step 3 : Find the concentration of Ca(OH)2 using its moles and volume.
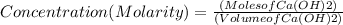
Moles of Ca(OH)2 = 0.002997 mol and volume of Ca(OH)2 = 0.027 L
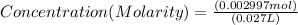
Concentration of Ca(OH)2 = 0.111 mol/L or 0.111 M