Hello!
When the pressure of a gas doubles, the new volume halves
Why?
This can be solved by assuming that the temperature remains constant and using the Boyle's Law, which states that pressure is inversely proportional to volume.
When there's a change in pressure:
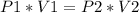
Solving for V2:
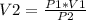
And
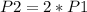 , so:
, so:
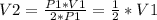
That means that the new volume is half the initial one.
Have a nice day!